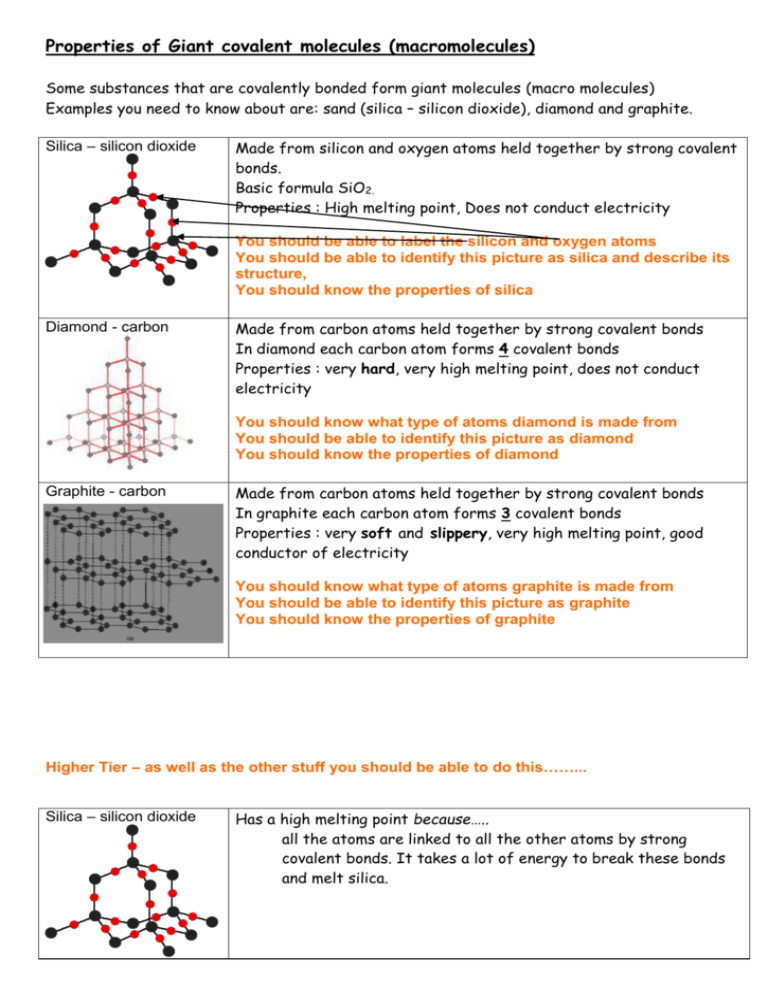
Does Silicon Dioxide Have a High Melting Point
Silica or silicon dioxide which is found in sand has a similar structure to diamond so its properties are similar to diamond. This is due to the strength of Si-O-Si binds in the lattice.

Based On Their Structures Explain Why Sodium Oxide Silicon Dioxide And Carbon Dioxide Have Different Melting Points Here Are 6 Real Student Answers Ppt Download
Silicon dioxide has a very high melting and boiling point because of the huge nummber of strong covalent bonds that require a lot of energy to be broken.
. Does silicon dioxide have a high or low melting and boiling point. What is silicon dioxide an example of. Covalent bonds so a lot of energy is needed.
Silicon has a very high melting point due to its giant covalent structure. Why does silicon dioxide have a high melting point. A giant covalent molecule.
High melting and boiling point. Argon exists as individual atoms with weak van der Waals forces between them which again results in a low melting temperature. 43 Votes The bond energy of Si is generally considered to be lower than that of the C-C so a simple explanation is that diamond has a stronger bond.
A lot of energy is needed to break the strong covalent bonds throughout the structure. Sulphur trioxide SO3 meanwhile has a simple covalent structure with no other bonds between molecules. SiO2 has a high melting point 1700 degrees Celsius.
Silicon dioxide has a high melting point - varying depending on what the particular structure is remember that the structure given is only one of three possible structures but around 1700C. Why does silicon dioxide have a high melting point. Silicon Dioxides melting point is 1710 C Whereas Diamond is about 4226 C However it usually does not melt but sublimes and sometimes just convert itself to graphite.
Silicon has a very high melting point due to its giant covalent structure. Silicon does not melt congruently to give a liquid of the same composition it decomposes at around 2700 0C. It will only melt and liquefy at a specific pressure.
Explain why Silicon Dioxide has a higher melting point than Sulfur Trioxide. 495 2724 Views. This means that it forms thousands of covalent bonds between its silicon and oxygen subunits.
Covalent bonds are much stronger than Van der Waals forces and. It is hard and has a high melting point but. Silicon dioxidesilicasand contains the elements silicon and oxygen covalently bonded together.
Silicon dioxide SiO2 has a macromolecular structure. A lot of energy is needed to break the strong covalent bonds throughout the structure. Why does silicon have a high melting point.
What needs to be broken when heating silicon dioxide. Why does silicon dioxide have a higher melting point than sulphur. Sulfur Trioxide has a simple molecular structure meaning it has Van der Waals forces between molecules.
Silicon dioxide also known as silica is an oxide of silicon with the chemical formula SiO 2 most commonly found in nature as quartz and in various living organisms. As you heat silicon up the crystal structure changes. Silicon Dioxide has a macromoleculargiant covalent structure which means it has covalent bonds between all atoms in its structure.
In many parts of the world silica is the major constituent of sandSilica is one of the most complex and most abundant families of materials existing as a compound of several minerals and as a synthetic product.
![]()
Macromolecules Covalent Network Solids Last Part Of Topic Ppt Download

Why Is Carbon Dioxide A Gas While Silicon Dioxide A Solid Quora
No comments for "Does Silicon Dioxide Have a High Melting Point"
Post a Comment